
 |
Service
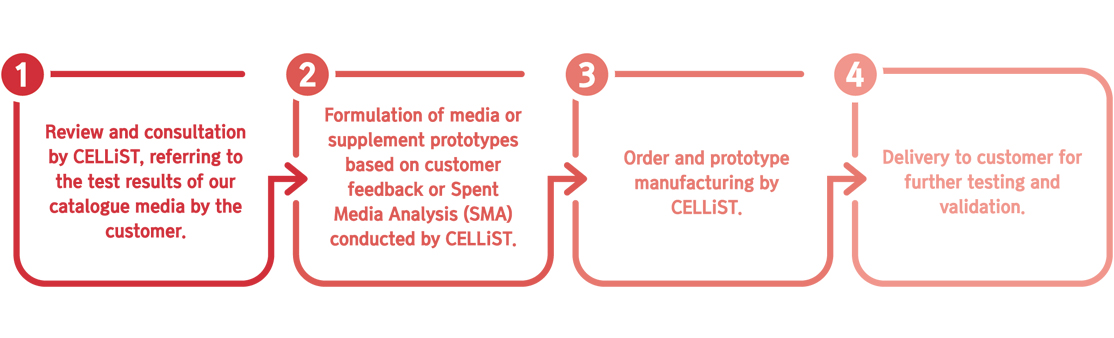
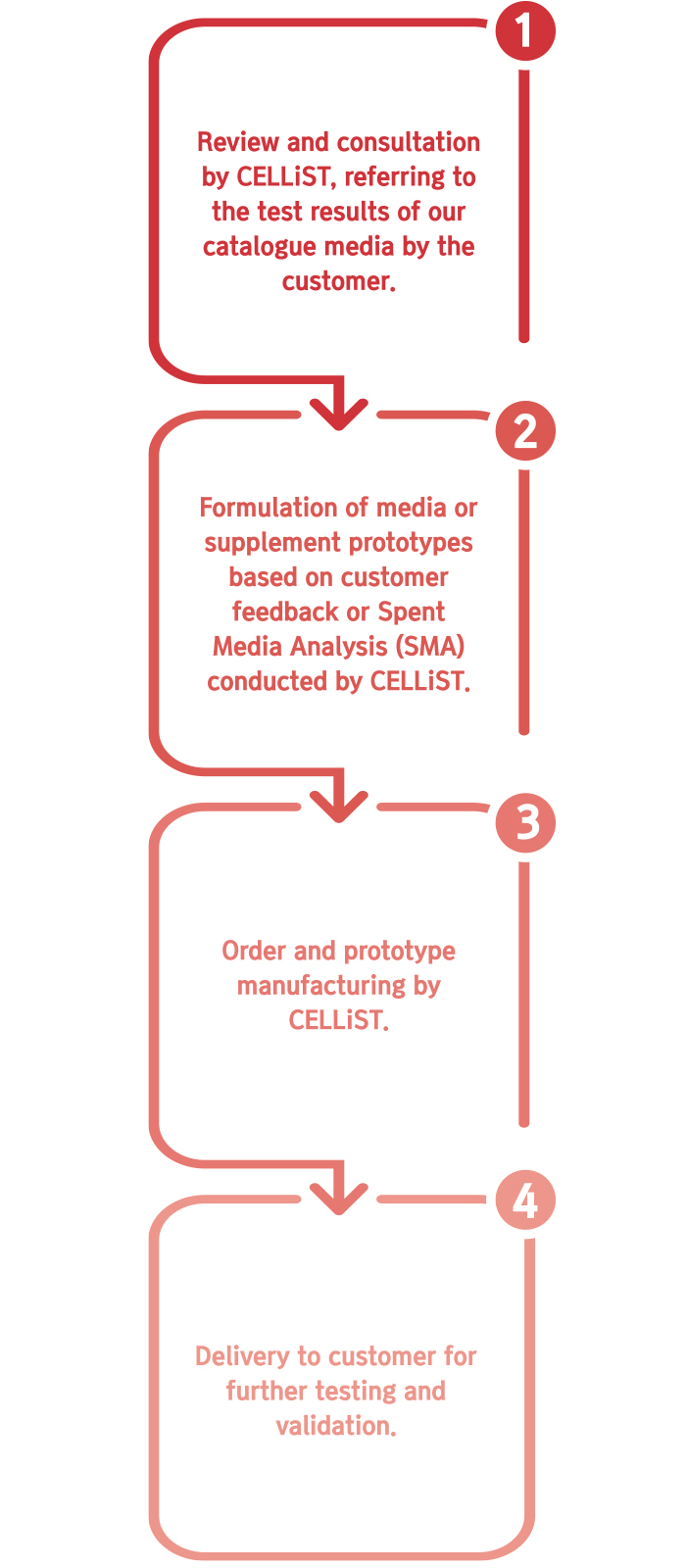
- 세포 배양 성능 및 공정 효율성을 향상시키기 위한 커스텀 배지 및/또는 보충제 제작
(Non-GMP 배지 프로토타입).
- 고객 요구 사항에 따른 유연한 생산 규모 대응.
- 신속한 프로토타입 제작 및 납품, 일반적으로 영업일 기준 10일 이내 완료.


• 분석 결과 (RoA): 맞춤 배지의 품질과 성능을 보장하기 위해 제공됨.
• 시험 항목: 외관, 용해도, pH, 삼투압 포함.
• 선택적 시험: TAMC/TYMC, 엔도톡신, 마이코플라스마, 세포 배양 시험 가능 (추가 리드타임 소요될 수 있음).
• Spent Media 분석(SMA) 데이터: 당사 카탈로그 배지를 사용한 초기 실험 결과(생존 세포 밀도(VCD), 생존율, 배양 중 대사물질 농도 등)를 제공하는 것을 권장함.
단백질 적정 농도(titer) 데이터는 선택 사항임.
• Spent Media 샘플: 고객이 실험 후 사용한 배지 샘플을 CELLiST에 보내면, 당사가 모든 대사물질 및 아미노산 농도 변화를 측정하여 종합적인 SMA 분석 수행 가능.
• 추가 정보: 고객의 세포주 및 공정과 관련된 특정 요구 사항이나 제약 사항을 공유하면 맞춤형 배지 프로토타입을 더욱 효과적으로 설계할 수 있음.