
 |
Service
아지노모도셀리스트코리아는 아지노모도㈜의 아미노산 기술과 30년 이상 지속해 온 동물 세포 배양 배지 사업에 대한 경험을 바탕으로 Media Customization 개발은 물론 기존 Media에 Supplement를 추가하여 Media 성능을 높이는 Fine Tuning 까지, 귀사의 세포주와 공정에 적합한 Media 를 제공하기 위한 다양한 Customizing Service를 제공하고 있습니다.


효율적인 배지 최적화를 위한 3가지 구성 성분 그룹화
세포 배양 배지는 약 70가지 성분으로 구성되어 있어 모든 성분을 최적화하는 것은 비현실적입니다. CELLiST는 배지 성분을 3가지 그룹으로 분류하여 효율적인 배지 최적화를 가능하게 하며, 이는 고객의 성공을 위한 핵심 전략이 됩니다.


초기 개발 단계뿐만 아니라 후기/상업 생산 단계에서도 최적화된 배지를 제공합니다.
상업 생산 단계에서도 제품 품질을 유지하면서 생산성을 향상시키고 비용을 절감할 수 있도록 돕는 Switch Program을 제공합니다.


| Stage | R&D | Clinical | NDA | Marketed |
|---|---|---|---|---|
| Pipelines | 27 | 21 | 1 | 4 |
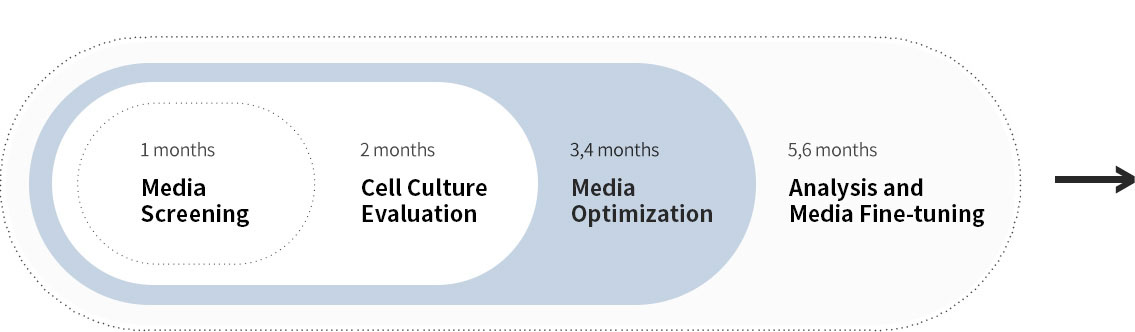
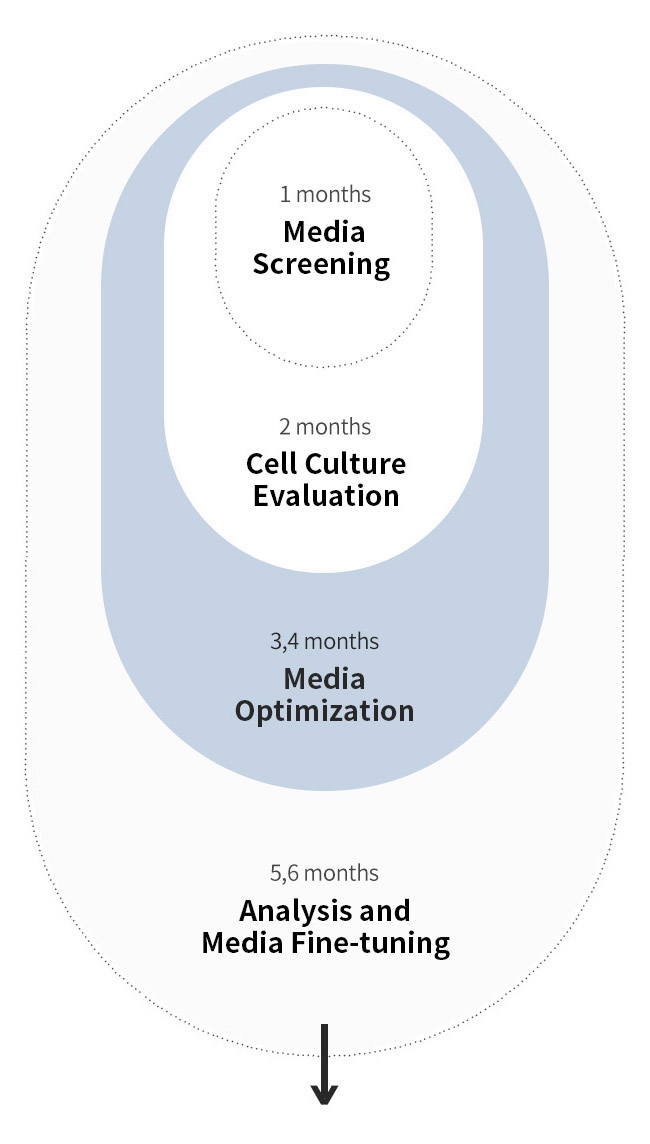
새롭게 확립된 CHO 세포주의 신속한 분석 후 집중적인 배지 개발 과정을 거쳐, 단기간 내 생산성이 크게 향상되었습니다.
당사의 독자적인 역량은 고객에게 강력하고 유연한 지원을 제공합니다:
고객 세포주에 최적화된 초기 배지를 통해 신속하게 개발을 시작할 수 있습니다. 다양한 산업 선도적인 high-throughput 시스템을 활용하여 후보 배지를 빠르게 평가할 수 있으며, 숙련된 연구진이 이를 지원합니다. 또한, 아미노산, 비타민, 미네랄 등 다양한 성분을 포함한 spent media의 정밀한 고속 분석을 통해 맞춤형 배지 최적화를 효과적으로 수행합니다. DoE 및 통계 분석을 당사의 오랜 배지 조성 개발 경험과 결합하여 최적의 배지 구성을 신속하게 설계합니다. 고객의 목표와 마감일을 고려하며, 당사의 전문 개발팀이 상업화 단계까지 전 과정에서 고객을 적극적으로 지원합니다.
Chugai사와의 협력을 통해 효율적인 배지 개발 및 최적화가 이루어졌으며, 생산성이 획기적으로 향상되어 단백질 농도 12 g/L의 높은 적산량(titer)에 도달하였습니다. 개발 과정에서 고농도 및 액상 안정성을 가능하게 하는 당사의 독자적인 L-Cysteine 기술이 적산량의 큰 향상에 기여하였습니다. 또한, 내부 노하우*와 추가적인 spent media 분석을 기반으로 칼륨 및 인산염 등의 농도를 최적화하여 배지 성능을 더욱 향상시켰습니다.


침전 억제

당사의 독자적인 배합이 L-시스테인의 안정성을 높여 액상 배지를 안정화시킵니다.
수율 향상

액상 안정성이 뛰어난 고농도 L-시스테인이 수율을 향상시켰습니다.