
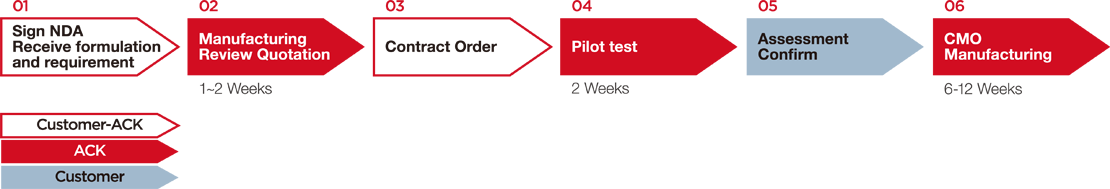
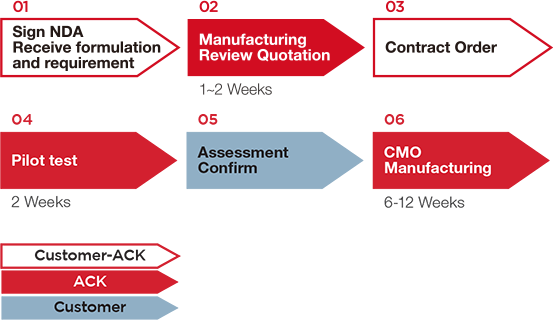
 |


Powder Media/Supplement: powder lots to 10 kg (non-GMP), to 1,000kg (GMP)
| Container (L) | 5L | 10L | 20L | 80L | 250L | 1,000L | 2,000L |
|---|---|---|---|---|---|---|---|
| non-GMP | ~2kg | ~5kg | ~10kg | ||||
| GMP | ~10kg | ~50kg | ~100kg | ~450kg | ~1,000kg |
Annual maximum production capacity: 200 ton (1 ton x 200 batches)
Various kinds of formulas includes:
Customer-developed formulas
Classical formulas with or without modifications (e.g. DMEM with low NaCl)
Media are packaged in aluminum pouch or plastic pail by weight
| Aluminum pouch | 6L Plastic drum | 20L Plastic drum | 60L Plastic drum | |
|---|---|---|---|---|
| Amount(kg) | ~0.050kg | ~3kg | ~10kg | ~20kg |
| Amount(L) | ~1L | ~50L | ~500L | ~1,000L |
Product quality are evaluated by the following tests
Result of Analysis is available for prototype.
Testing includes Appearance, Solubility, pH and Osmolality.
TAMC/TYMC, Endotoxin, Mycoplasma and Cell culture are optional, requiring more lead-time to the delivery.